Background:
Transferrin Receptor (TfR) 1 is thought to be anti-cancer target by controlling ion supply for decades. Anti-TfR1 fully human monoclonal antibody (PPMX-T003) has an uniqe epitope on the ligand-binding domain of TfR1. This antibody can efficiently inhibit iron influx into cells rather than preceding reported similar antibodies (US20220332838). This antibody can inhibit growth of erythroblast as well as various cancer cell lines at subnanomolar level, both cells are highly expressed TfR. Antibody administrated on normal cell expressing TfR at low levels has little effect except for RBC formation. Prior to apply cancer therapy, the safety was evaluated at low dose in healty volunteers (Ogama et al., 2023). In this study, we aimed to determine safety profiles at higher dose in patients with polycythemia vera (PV) by inhibiting elevation of erythropoiesis, followed by P1a study. Except for an antibody drug conjugate (CX-2029), this is the first-in-class trial of an unmodified human antibody other than the murine P1a trial conducted in the 1990s.
Methods:
This is a multicenter, open-label intra-patient dose escalation study (NCT05074550). Adults patients are eligible who were diagnosed with PV and had periodical phlebotomy (PLB) between 4 to 9 weeks. Patients were excluded if they had cytoreductive therapy. The primary objective is to evaluate the safety and the pharmacokinetics after single dose of administration. The secondary objective is pharmacodynamics, such as RBC count, hemoglobin, hematocrit. Dose escalation is an intra-patient dose escalation design, due to disease progression being individually different in each patient. Starting dose (0.25mg/kg) was determined as previous P1a trial in healty volunteers (n=40) observed decreased hemoglobin level out of normal value. A single dose of PPMX-T003 is intravenously infused for 1 hour, one day after a scheduled PLB. Dose is increased (0.25, 0.4, 0.64, 1mg/kg) when the next PLB treatment scheduled within 4 to 9 weeks, depending on the participants' adverse event (AE) profiles. The primary endpoint is the incidence and severity of treatment-related AEs, Key secondary end point meets next PLB does not required for more than 12 weeks.
Results:
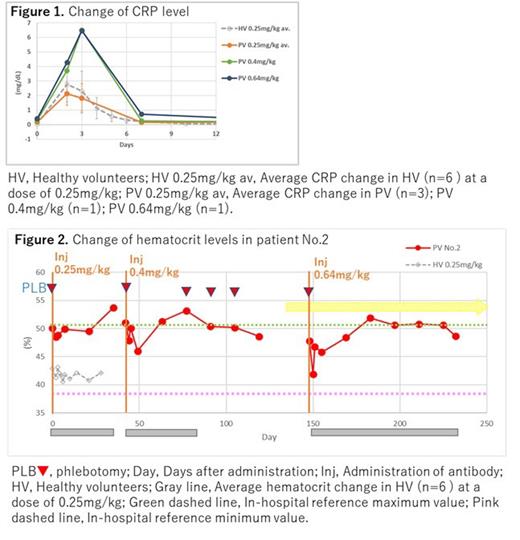
As the data cutoff at the end of June 2023, all three cases did not require PLB for more than 12 weeks as reached endpoint. Two cases did not require PLB immediately after the first single dose of 0.25mg/kg. Both cases were observed no AE. Another patient was required periodical PLB within 4 to 9 weeks interval at two consecutive doses of 0.25-0.4mg/kg, then escalated up to the third dose of 0.64mg/kg till reached end point with mild AE, which was significant fatigue and mild fever, up to 37.6℃ within 24 hours after the highest dose of infusion, approximately 6.5 mg/dL CRP elevation after 3days after infusion, lymphopenia 2days after infusion recovered after 7 days. Those AEs are considered to be standard Infusion Related Reactions observed in other antibody therapeutics. Hematocrit and hemoglobin were controlled below 50% and 15.5g/dL, respectively, while RBC count are rather elevated up to 7 million/mL and MCV was down to 73 fL. Pharmacokinetics revealed half-life of the antibody was up to 12hrs, which is shorter than other antibody therapeutics. Enrollment is on going.
Conclusions:
AEs are mild as observed within the level of previous healthy volunteer's trial. Efficacy observed in all erythroid parameters including hematocrit, hemoglobin and other parameter shown symptoms seen in iron deficiency anemia. This antibody can specifically suppress erythrocyte differentiation by inhibiting iron influx in the target erythroid lineage, which cells are highly expressed TfR1, without any significant AE on other normal organs with low expression level of TfR. PPMX-T003 affects on erythrocyte differentiation in bone marrow may expect to reduce PLB treatment clinically. This preliminary result could be given helpful safety prospect recently initiated P1/2 trial in patients with aggressive NK leukemia (NCT05863234), supported by a grant from the Japan Agency for Medical Research and Development (23nk0101231j0002).
References:
Ogama, Y. et al., Phase 1 Clinical Trial of PPMX-T003, a Novel Human Monoclonal Antibody Specific for Transferrin Receptor 1, to Evaluate Its Safety, Pharmacokinetics, and Pharmacodynamics. Clin Pharmacol Drug Dev. 2023;12(6):579
Disclosures
Ito:CSL Behring: Honoraria; Eisai: Honoraria; Nippon Shinyaku: Honoraria; AbbVie GK.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Novartis: Honoraria; Mundipharma: Honoraria; Kyowa Kirin: Research Funding; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Asahi Kasei Pharma Corporation: Research Funding; Bristol-Myers Squibb Company: Honoraria, Research Funding; Sanofi: Honoraria. Nakamae:Meiji Seika Pharma: Research Funding; Bristol-Myers Squibb: Research Funding; Alexion Pharma: Research Funding; Parexel International Inc: Research Funding; CMIC Company: Research Funding; Sumitomo Dainippon Pharma: Honoraria; Amgen: Honoraria; Takeda Pharmaceutical Company: Honoraria; DAIICHI SANKYO COMPANY: Honoraria; Novartis: Research Funding; Janssen Pharmaceutical K.K.: Honoraria; Abbvie: Honoraria; Nihon Shinyaku: Honoraria; Otsuka: Honoraria; Astellas: Honoraria. Matsuura:Perseus Proteomics inc: Current Employment, Patents & Royalties: I'm one of inventor of patents for Ab therapeutics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal